- TOP
- Businesses
- Characterization Analysis
Characterization Analysis
Contract Services for Characterization Analysis of Cell and Gene Therapies
At Cyto-Facto Inc., we offer contract services for the characterization analysis of gene and cell therapies. Our flexible support tailored to meet customer needs, including the establishment of testing systems and proposals for QC testing at GMP levels. We can also accommodate requests based on reliability assurance standards.
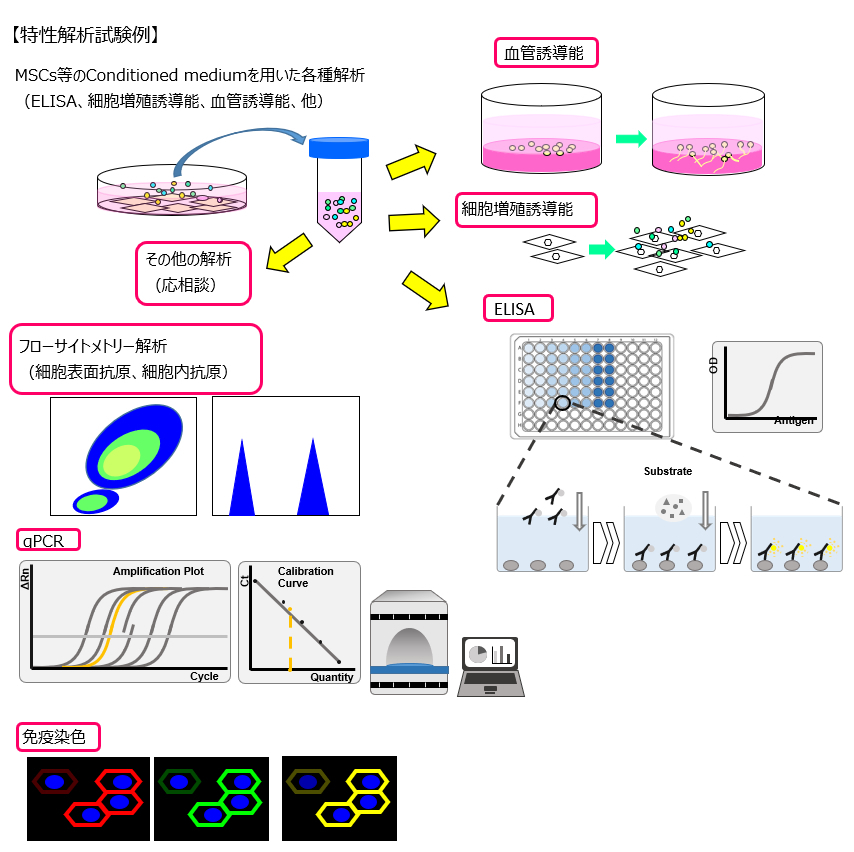
Examples of Characterization Analysis Tests
In addition to CAR-T, MSC, and iPSC, we welcome inquiries for any desired cell types. If there are analysis methods not listed in the examples, please feel free to contact us for further consultation.
CAR-T
■Cell surface antigen analysis (Flow Cytometry)
■Residual virus testing (ELISA, ddPCR, qPCR)
■In vitro tests (IL-2 dependent proliferation test, cytotoxicity assay)
■In vivo tests (Efficacy & pharmacology tests, toxicity tests, biodistribution tests)
MSCs
Clinical trials, reliability assurance standards (autologous)
■Cell surface antigen analysis (Flow Cytometry)
■In vitro tests (Differentiation induction, etc.)
■Stability testing
Non-Clinical (Allogeneic)
■Cytokine quantification (ELISA)
■In vitro tests (T-reg induction)
■In vivo tests (Anti-inflammatory tests)
ESC/iPSC
■Cell surface antigen analysis (Flow Cytometry)
■Evaluation of trilineage differentiation potential (Embryoid body formation, Socerecard panel, qRT-PCR)
■In vitro tests (IL-2 dependent proliferation test, cytotoxicity assay)
■In vivo tests (Tumorigenicity tests, immunohistochemical staining)
