- TOP
- Businesses
- CMO/CDMO
CMO/CDMO
Contract Manufacturing Services for cell & gene Therapies
Cyto-Facto Inc. has accumulated technology, expertise , and clinical trial experience through the clinical and commercial manufacturing of CAR-T cells and mesenchymal stem cells (MSC). With experienced personnel, facilities, and equipment capable of supporting all phases from basic research to commercial production, we can provide comprehensive support from the early stages of development to commercial manufacturing.
Clinical Trials and Commercial Manufacturing

Under a system compliant with PIC/S GMP and GCTP, as well as other various requirements, we handle everything from establishing manufacturing procedures to producing regenerative medicine products. By implementing a thorough Quality Management System (QMS) and managing quality risks through Quality Risk Management (QRM) for activities that may affect quality, we ensure the constinuous production of high-quality products at all times.
・Clinical and commercial manufacturing based on PIC/S GMP
・Establishment of clinical manufacturing procedures based on research-grade protocols (upgrading to GMP level)
・Development of scaling-up procedures from clinical to commercial manufacturing
・Investigation of compliance with biological raw material standards and selection of manufacturing equipment
・Quality Risk Management (QRM) based on PIC/S GMP and ICH Q9
【Examples of Achievements】
・Clinical and commercial manufacturing of CAR-T cell therapy "Kymriah® infusion"
(Conducted by the predecessor, the Foundation for Biomedical Research and Innovation at Kobe)
At that time, it was the only CAR-T manufacturing site in Asia and the first of its kind in Japan.
・Clinical manufacturing of autologous synovial mesenchymal stem cells (Development Code: FF-31501)https://www.fujifilm.com/jp/ja/news/list/9142
Contracted by FUJIFILM Corporation to manufacture clinical trial products for FF-31501, used in a Phase III clinical trial for meniscus injury.
Process Development
We support process development across various phases, from preclinical to clinical stages.
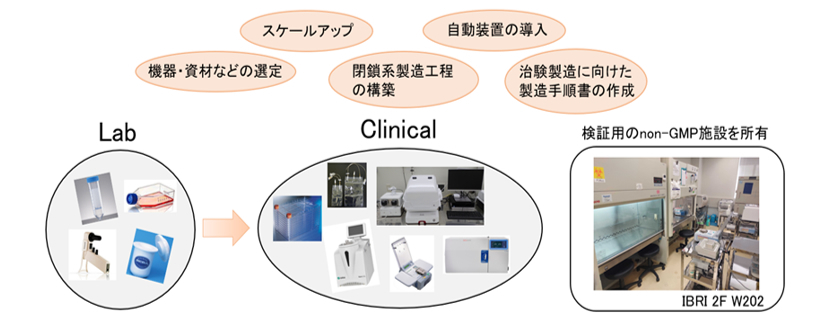
【Examples of Implementation】
・Design and execution of preclinical trials
・Creation of GMP-grade manufacturing SOPs based on research-grade protocols
・Consideration of the introduction of automated culture systems
・Consideration of transitioning open system operations to closed system processes
・Scale-up of culture systems and evaluation of large-scale culture methods
・Verification and other activities in the process development room (non-GMP)
【Examples of Installed Equipment and Facilities】
・Closed system culture equipment
・Automated culture equipment
・Non-GMP development room
Manufacturing Site
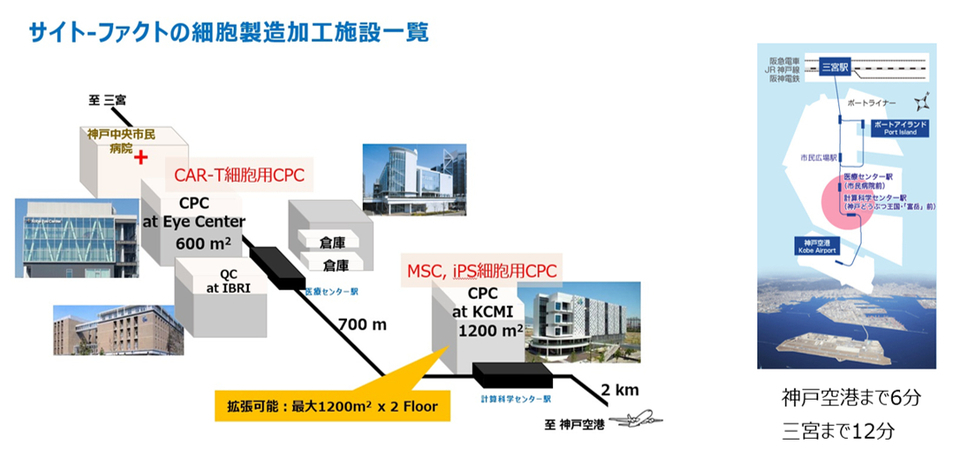
Cyto-Facto has a facility on Port Island in Kobe City, Hyogo Prefecture, capable of conducting manufacturing and analysis of CAR-T, MSC, and iPSC.

■KCMI

IT Management of Manufacturing Processes
At Cyto-Facto, we are committed to enhancing quality assurance through the efficiency of creating, reviewing, and approving manufacturing records, reducing the risk of human errors, and ensuring data integrity in cell and gene therapy product manufacturing. To achieve this, we have introduced a cloud-based electronic batch record system, BatchLine.
Additionally, we are working on fully digitizing all processes, including the receipt and inventory management of raw materials, manufacturing processes, and product shipments. For this purpose, we are developing an integrated manufacturing management system, Cytofactory 4.0™ (CF4.0™), in-house.
