- TOP
- Businesses
- CytoFactory 4.0
CytoFactory 4.0

Release Scheduled for April 2025!
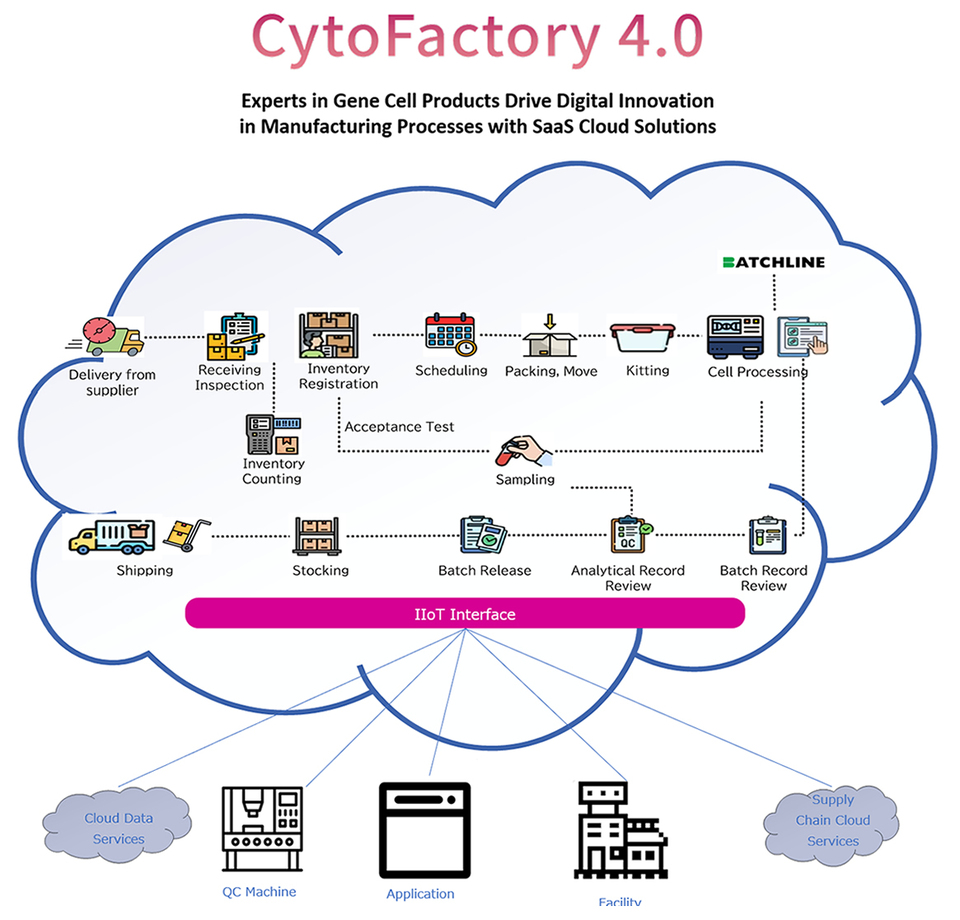
Key Features
Optimizing Workload with RFID Management
・Reduce workload through RFID management of raw materials, kitting, and final product verification across ambient, refrigerated, and frozen conditions. Streamline your processes for greater efficiency and accuracy.
Enhancing Efficiency with a Paperless Approach
・Revamp your operations by eliminating thousands of paper-based tasks through digitization of records and management processes. This transition leads to a remarkable reduction in product delivery cycle times to patients, enhancing overall efficiency and service quality
Centralized Master Data Management
・Mastering and centrally managing all aspects, including raw materials, kitting, manufacturing processes, QC samples and testing, as well as quality evaluation and shipping.
Seamless Integration Capabilities
・Experience robust connectivity with applications like manufacturing equipment, testing devices, and procurement systems. Our platform seamlessly integrates with systems such as Quality Management Systems (QMS) and supply chain management tools, ensuring streamlined operations and enhanced efficiency
Digitalizing SOPs and Workflows
・Transform your SOPs and business workflows into digital formats using MBR and Process Builder. Enhance efficiency, ensure compliance, and streamline operations with our electronic solutions
