- TOP
- Businesses
- Consultation
Consultation

Services
We support domestic and international pharmaceutical companies and biotech ventures in solving challenges at each phase of cell and gene therapy development and manufacturing.
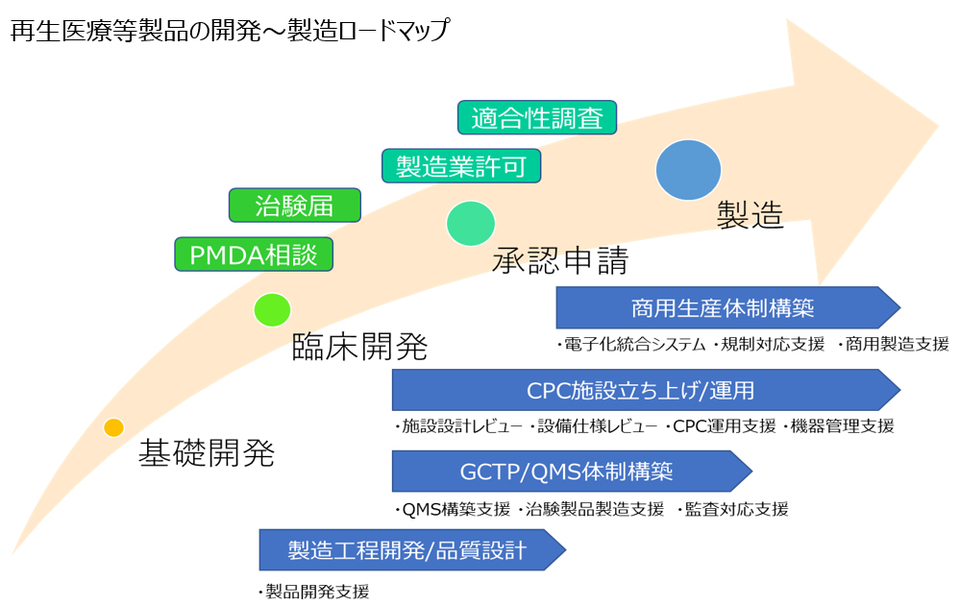
Product Development Support:We support the process from development seeds to the manufacturing of clinical trial products.
Regulatory Compliance Support:We assist with compliance to various regulations.
QMS Construction Support:We support the establishment of pharmaceutical quality systems.
Audit Response Support:We help with responding to GCTP conformity inspections.
Facility Design & Specification Review Support / CPC Operation Support:We provide support from the design to the operation of CPC facilities.
Commercial Manufacturing Support / Clinical Trial Product Manufacturing Support / Electronic Integrated System:We support the development of manufacturing systems and the digitization of records.
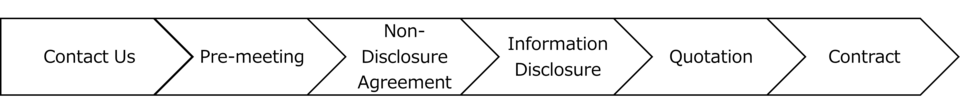
Flow of Service
Service Catalog

・Product Specification Design Support
・Quality Management Strategy Development Support
・Manufacturing/Test Method Development Support
・Technology Transfer Support
・Aseptic Operation Operator Training

・Cartagena Application Support
・GCTP Compliance Investigation Response and Inquiry Support
・Gap Analysis between GMP/GCTP and Your Company's Current Status
・Business License Application Support
・Support for Developing and Maintaining Manufacturing SOPs and QC Testing SOPs
・QMS SOP Creation Support (Deviations, Changes, Recalls, Quality Information, Self-Inspection, Training, Documentation, etc.)
・Support for Building GCTP Organization

・Mock Audit
・Audit Response Training

・Facility Design Review
・Equipment/Facility Acceptance Testing Plan and Report Review
・Validation Plan and Report Review
Equipment Management Support
・Calibration and Maintenance Plan/Report Review
Calibration and Maintenance Plan/Report Review
・Support for Developing Hygiene Management Programs
・Operational Improvement Support

・Support for the Development/Improvement of Pharmaceutical Quality Systems
・GMP Training
・DI Training
・Supplier Management Agency and Support
・Self-Inspection Agency and Support
・Support for Raw Material Management
Support for Clinical Trial Product Manufacturing
・GMP Training for Investigational Drugs
・DI Training
Electronic Integrated System
・Support for the Implementation of an IT Integrated Manufacturing Management System
・Consulting for the Development of Automated Culture Equipment
Achievements
Autologous Synovial Mesenchymal Stem Cells
・Preparation of Manufacturing Procedures
・Preparation of Quality Control Test Procedures
・Supplier Management Agency
・Raw Material Management
・Preparation of Validation Plans
