- TOP
- Strength
strengths of CF
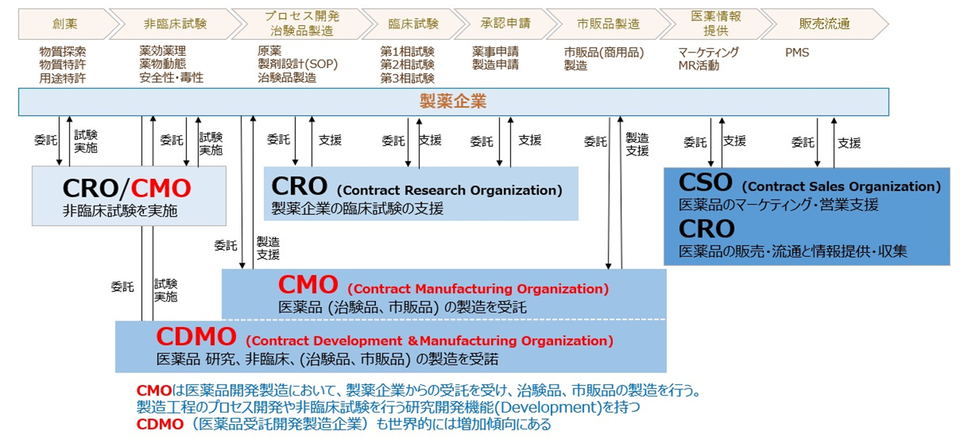
One-stop manufacturing through high-quality and extensive services
As the first commercial manufacturing site in Asia for the world’s first cell and gene therapy product, Kymriah® (CAR-T cell intravenous infusion), Cyto-Facto has accumulated extensive experience in manufacturing expertise and know-how, under GMP management Our experienced staff are dedicated to manufacturing, quality control, and quality assurance across all phases, from basic research to commercial product manufacturing.
We possess both hardware and software that comply with the standards for manufacturing management and quality control of regenerative medicine products (GCTP regulations), providing comprehensive high-quality services from early development to commercial product manufacturing.
International-Standard Manufacturing Established Through Commercial Production of CAR-T for Global Mega-Pharmaceutical Companies
Consulting by Experienced Staff with Extensive Experience in Basic Research

Cyto-Facto is comprised of 64 employees, including 9 with PhDs and 22 with master's degrees, and has a strong focus on the "D (Development)" function as a Contract Development and Manufacturing Organization (CDMO). Based on our experience in the commercial manufacturing of cell and gene products, we provide practical and hands-on consulting services.
Process Development Aimed at Commercial Product Manufacturing

Leveraging the experience and expertise gained from the commercial manufacturing of CAR-T products, we upgrade laboratory-level protocols to manufacturing SOPs. Our services offer a wide range of services, including scale-up, selection of equipment and materials, construction of closed systems, implementation of automation devices, and creation of manufacturing SOPs. Additionally, we also have non-GMP facilities available, allowing for verification and other related activities.